
An exceptional 9.89 carats photochromic zircon
We were fortunate to observe and analyse a remarkable 9.89 ct zircon showing a strong change of colour from vivid orange to dark brown after LWUV illumination. Here we report the first analyses of one of the largest zircons with T-type photochromism documented.
In the 1970s, what is now the Swiss Gemmological Data Foundation (SGDF) received as a gift a remarkable brown zircon weighing around 9.89 carats (≈ 10.08x10.95x7.31 mm; Figure 1) of unknown origin. Identification of this gem was confirmed by Raman spectroscopy. Microscopic observation revealed relatively marked colour zonation (brown to almost colourless) and the presence of clusters of small, elongated prismatic inclusions. Yet, due to their size, the exact nature of these inclusions remains undetermined. This massive zircon aroused curiosity when it was observed that its colour changed, becoming lighter after several months' storage in the dark. Exposure to visible light seemed to have the opposite effect, gradually darkening the colour. This phenomenon corresponds to a property identified in other gems such as sodalite-hackmanite or chameleon diamonds: the photochromism (Blumentritt & Fritsch, 2021, 2022).
Photochromism is a rare optical property characterised by a reversible colour change following exposure to electromagnetic radiation - most often ultraviolet light (Bouas-Laurent & Dürr, 2001). The reversibility is achieved either by exposure to another electromagnetic radiation (e.g., daylight), or by the action of temperature (heating of the sample). These two conditions for returning to the initial state are known respectively as P-type photochromism and T-type photochromism (Bouas-Laurent & Dürr, 2001).
In our case, this remarkable zircon shows a relatively dark orange-brown colour in daylight and handled under normal observation conditions (ambient temperature ≈ 20°C, LED illumination 3600K; Figure 1 centre). However, this state is considered as intermediate. Indeed, when exposed to UVL (365 nm) for a few seconds, the colour turns dark brown (Figure 1, right). No noticeable luminescence was observed when the zircon was exposed either to UVL or UVC using a hand-held UV lamp. The brown colour photogenerated by UVL corresponds to the excited or metastable state. Return to the stable state is achieved by thermal de-excitation. The brown colouration disappears after several months in the dark, the ambient temperature giving enough energy for the return to the stable state and the absence of light preventing excitation. The brown colouration can also disappear in a few seconds when the sample is heated at about 100-110°C with a heat gun, forcing a return to the stable state. In this stable state, the zircon then displays a vivid orange colour (Figure 1, left). Finally, when the stone cools down to room temperature, the artificial lighting of a room is enough for it to return to its intermediate orange-brown state.

These observations therefore correspond to T-type photochromism and were defined in a previous article as type 2 of the 3 photochromic behaviours observed in zircons (Blumentritt & Fritsch, 2021). The absorption spectra of the two* – stable and metastable – are also consistent with those observed in other samples with the same photochromic behaviour (Figure 2). There are uranium-related absorptions (654 and 691 nm) that do not appear to be affected by the photochromic reaction. On the other hand, the absorption of the broad bands at 510 and ~800 nm decreases and increases respectively as the colour changes from orange to brown. While the absorption band at 510 nm is attributed to a complex colour centre linked to yttrium (Zeug et al., 2018), the broad band around 800 nm remains unexplained.
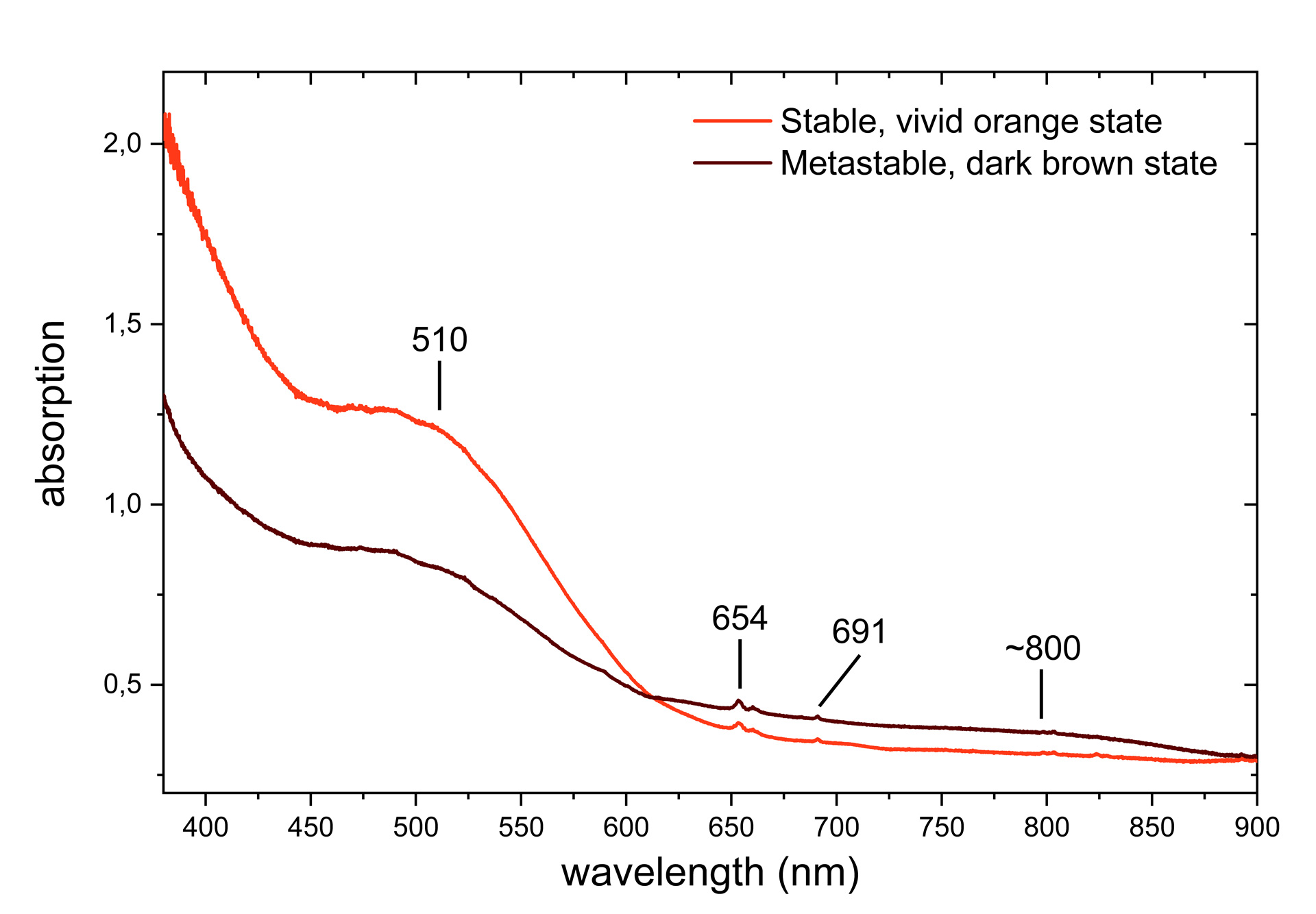
Although the photochromism of zircons has already been described in the literature (Koivula & Misiorowski, 1986; McClure, 2011; Renfro, 2016; Suthiyuth, 2014), the explanation for the colour change is still incomplete. By comparison with other photochromic gems (see for example: Byrne et al., 2014; Claffy, 1953; Kondo & Beaton, 2009; McClure et al., 2005), in view of the energies involved in the reaction (UVL radiation, thermal energy ≈ 100°C) and the impact on the absorption spectrum in the visible range, it is likely that an electronic transfer is the source of the photochromism in zircon. However, it remains to be defined which are the electron donor and acceptor centres. Given that zircon is not intrinsically photochromic, these are probably intrinsic or extrinsic defects such as substitutions, interstitial atoms, vacancies or more complex combinations of these defects. The definition of these centres is currently the subject of further study by the two authors in an international collaboration (GGTL Laboratories, Switzerland; Nantes University, France; University of Vienna, Austria). The remarkable sample studied here is part of a batch of zircons exhibiting other photochromic behaviours that will be the subject of a more detailed study at a later date.
*The absorption spectra were recorded by placing the sample in an integrating sphere. The zircon was first heated to ~100°C and placed still warm in the sphere to keep it as orange as possible during spectrum acquisition. Without moving it, the zircon was then exposed to UVL for approximately 5 minutes after cooling to acquire the spectrum in its dark brown state.
Bibliographical references
- Blumentritt F. & Fritsch E. (2021) Photochromism and Photochromic Gems: A Review and Some New Data (Part 1). Journal of Gemmology, vol. 37, N°. 8, pp. 780–800, https://doi.org/10.15506/JoG.2021.37.8.780
- Blumentritt F. & Fritsch E. (2022) Photochromism and Photochromic Gems: A Review and Some New Data (Part 2). The Journal of Gemmology, vol. 38, N°. 1, pp. 80–92, https://doi.org/10.15506/jog.2022.38.1.80
- Bouas-Laurent H. & Dürr H. (2001) Organic photochromism (IUPAC Technical Report). Pure and Applied Chemistry, vol. 73, N°. 4, pp. 639–665. https://doi.org/10.1351/pac200173040639
- Byrne K.S., Chapman J.G. & Luiten A.N. (2014) Photochromic charge transfer processes in natural pink and brown diamonds. Journal of Physics Condensed Matter, vol. 26, N°. 23, 239502-1, https://doi.org/10.1088/0953-8984/26/3/035501
- Claffy E.W. (1953) Composition, tenebrescence and luminescence of spodumene minerals. American Mineralogist, vol. 38, N°. 11–12, pp. 919–931. https://doi.org/10.1016/0022-3697(94)00251-7
- Koivula J.I. & Misiorowski E. (1986) Undesirable color change in blue zircon. Gems & Gemology, vol. 22, N°. 3, pp. 188–189, https://doi.org/10.1007/BF01115777
- Kondo D. & Beaton D. (2009) Hackmanite/Sodalite from Myanmar and Afghanistan. Gems & Gemology, vol. 45, N°. 1, 38–43, https://doi.org/10.5741/GEMS.45.1.38
- McClure S.F. (2011) Tenebrescent Zircon. Gems & Gemology, vol. 47, N°. 4, pp. 314–315, http://dx.doi.org/10.5741/GEMS.50.2.151
- McClure S.F., Rossman G.R. & Shigley J.E. (2005) Tenebrescent scapolite from Afghanistan. Gems & Gemology, vol. 41, N°. 3, pp. 269–271.
- Renfro N.D. (2016) Reversible color modification of blue zircon by long-wave ultraviolet radiation. Gems & Gemology, vol. 52, N°. 3, pp. 246–251, https://doi.org/10.5741/gems.52.3.246
- Suthiyuth R. (2014) Tenebrescent Zircon. Gems & Gemology, vol; 50, N°. 2, pp; 156–157, http://dx.doi.org/10.5741/GEMS.50.2.151
- Zeug M., Nasdala L., Wanthanachaisaeng B., Balmer W.A., Corfu F. & Wildner M. (2018) Blue zircon from Ratanakiri, Cambodia. Journal of Gemmology, Vol. 36, N°. 2, pp. 112–132, https://doi.org/10.15506/JoG.2018.36.2.112
AUThoRS
- Féodor Blumentritt, GGTL Laboratories Switzerland. ResearchGate, LinkedIn.
- Franck Notari, GGTL Laboratories Switzerland. ResearchGate, LinkedIn
© Association Gemmologie & Francophonie. This article was published in "GEMMES". (2023), N° 1, pp 15-17.
