
Cobaltocalcite or cobaltoan calcite: A Swiss answer
Switzerland is not known for its production of gemstones but for being an important platform for trade, auctions and certification of gems. GemGenève trade fair second edition (held from the 9th to the 12th of May 2019) has brought into light a new Swiss gem material in the designer corner. Geneva-based art jeweller Grégoire Maret (Pierre d'Alexis S.A.) presented this pink to purplish-pink cobaltoan calcite in his hand-made creations (Figure 1).

The "Rose of mine" is the fancy name given to this pink calcite (identified as calcite and not aragonite using infrared specular reflectance). This gem is rarely seen in high-end jewellery and is rather unique in its nature and geological origin. The gem calcite is coloured by traces of Co2+ in octahedral coordination in the calcite structure (Fritsch and Rossmann, 1987) and shows a variation in saturation and hues. The rough material, already described by Nicolas Meisser (Meisser 1999) is sporadically extracted from abandoned coalmines situated near the village of Isérables in the canton of Valais in Switzerland, from which some gemmy material is cut as cabochon. Unlike most gemstones in the trade today, this material is formed in very unique conditions.
This attractively coloured calcite crystallizes at ambient temperature and pressure through percolation of fluids enriched with cobalt, nickel and zinc (Meisser, 1999) forming speleothems on black shale host rock in the moist darkness of the abandoned mine shafts (Figure 2). Following the cessation of mining coal in 1943, surface waters interacted with the surrounding rocks and gave birth to this very contemporary gem.

Standard gemmological testing on this strongly birefringent polycrystalline material reveals an RI of 1.49-1.66 and a specific gravity of approximately 2.70. Only a very weak purplish luminescence is observed under LWUV (365 nm) and the material is inert under SWUV (254 nm). Under magnification, the polished cabochon stone shows granular to fibrous appearance and columnar growth with inhomogeneous colour distribution, the darker areas resulting from the presence of remnants of black shale host rock. The surface of the piece of uncut rough (Figure 3) exhibits trigonal prismatic crystals without preferential orientation resulting in the general botryoidal aspect of the surface and making the material tougher than monocrystalline calcite.

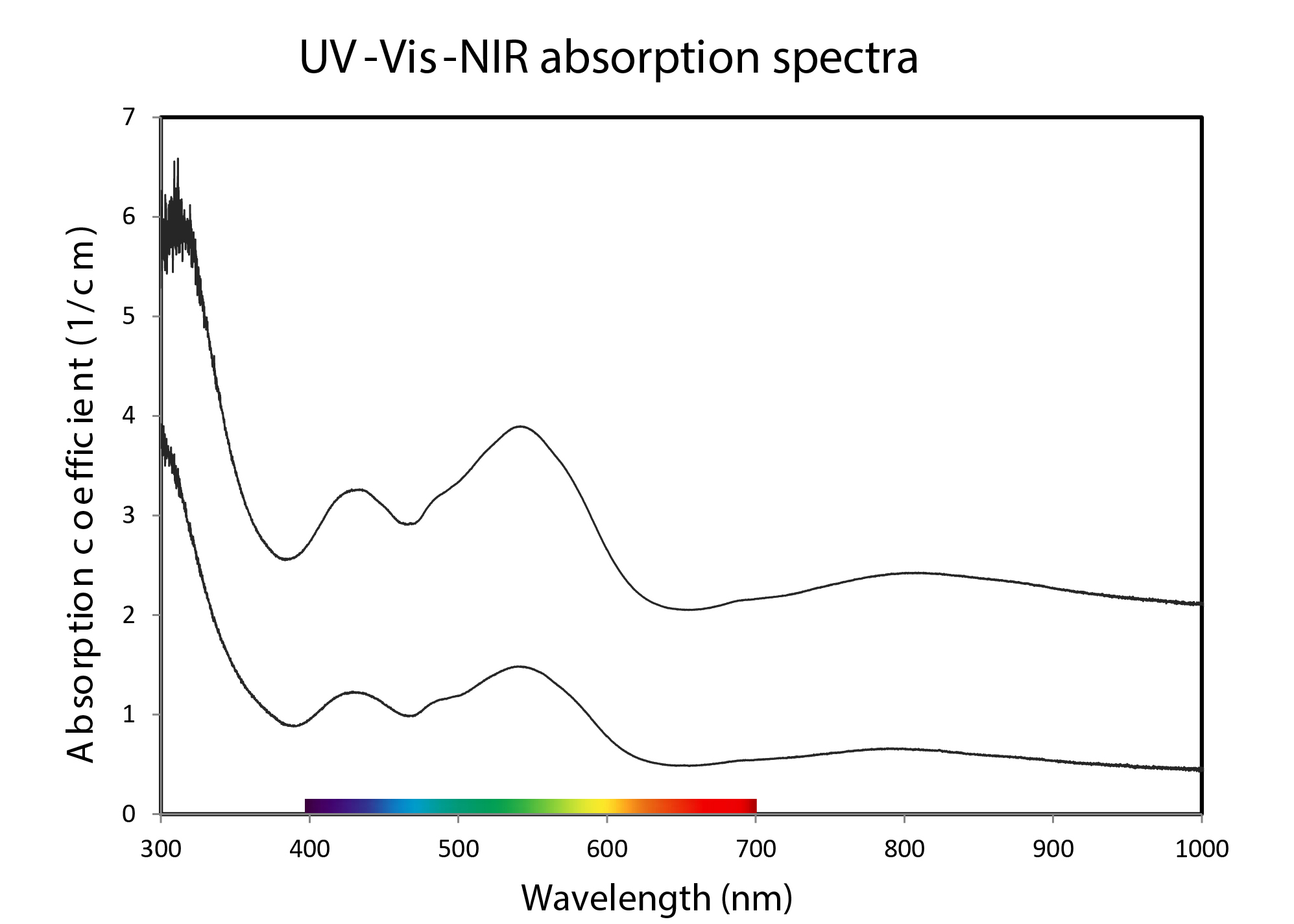
The UV-Vis-NIR spectra (Figure 4) obtained from this material exhibit the classic absorptions due to the presence of Co2+ with two broad bands with the dominant absorption centred at ≈ 535 nm and the secondary at ≈ 432 nm. Semi-quantitative analysis of trace element measured on zones with different colour saturation with EDXRF (Thermo Fisher’s ARL Quant’X) indicated the presence of between ≈ 850 and ≈ 1400 ppmw of Co and up to ≈ 220 ppmw of Ni in the studied samples. The pink colour in calcite is also known to be due to trace amounts of manganese, however, in this material manganese was not detected by EDXRF analysis in contrast with similar material studied by Siritheerakul and Sangsawong (2015).
The frequent use of the term cobaltocalcite is to be avoided to name calcite with traces of cobalt as a colouring agent ((Ca,Co)CO3). A rare carbonate of cobalt called spherocobaltite (Weisbach, 1877) exists and contains cobalt as the main component (CoCO3), which is not the case with the material presented here. It should then be called cobaltoan calcite.
Author
- Dr Eric May, GGTL Laboratories Switzerland. ResearchGate, LinkedIn.
- Franck Notari, GGTL Laboratories Switzerland. ResearchGate, LinkedIn.
- Nicolas Meisser, Musée Cantonal de Géologie, Quartier UNIL-Chamberonne, Bâtiment Anthropole, CH 1015 Lausanne, Switzerland.
References
- Fritsch, E. and Rossmann, G.R., 1987. “An update on color in gems. Part 1: Introduction and colors caused by dispersed metal ions.” Gems & Gemology, Vol. 23, No.4, pp. 126-139.
- Meisser, N., 1999. “La calcite cobaltifère d’Isérables, Valais.” Le Cristallier Suisse, 12/11, pp. 594-598.
- Siritheerakul and Sangsawong, 2015. "Pink and Reddish Purple Cobaltocalcite." Gems & Gemology, Vol. 51, No. 1, pp. 58-67.
- Weisbach A., 1877, Mineralogische Mittheilungen. - Jb. für das BergY und Hüttenwesen im Königreiche Sachsen, abh.: 42 - 53 (Sphäro-kobaltit)
© The Gemmological Association of Great Britain. This article was published in "The Journal of Gemmology". (2019), Volume 36, Issue 8, pp 685-686.
